
Division of Research and Economic Development
The Division of Research and Economic Development supports the campus community by providing a variety of resources and expertise to promote university research and sponsored programs.
Get to Know Our Division
Sponsored Programs
From identifying grant opportunities and writing grant proposals to ensuring research integrity and compliance, our office provides a variety of resources and expertise to promote university research and sponsored programs.


Research Integrity and Compliance
We ensure compliance with federal, state, and system regulations by providing consultations, education, and information to help the research community achieve the highest standards of ethical research conduct.
Economic Development
We facilitate economic growth in the community by cultivating and strengthening partnerships that serve the needs of the Northeast Texas region.

Export Control
We ensure integrity and compliance with export control regulations enacted by the U.S. government to control the export of sensitive equipment, software, and technology to protect our national and economic security.
Faculty Spotlight

Lucy Pickering, Ph.D.
Ted and Donna Lyon Center for Gamebird Research
The Ted and Donna Lyon Center for Gamebird Research seeks to revolutionize research, education and collaboration in order to foster sustainable gamebird populations in the state.

Upcoming events

Research Engagement
The Division of Research and Economic Development hosts a variety of events for the campus research community, including symposia that highlight undergraduate and graduate student research, and research training opportunities for faculty, staff, and students.
Research News
View All Research News
Saving the Quail: The Center for Gamebird Research at East Texas A&M is Restoring a Valuable Texas Resource
The Lyon Center for Gamebird Research at East Texas A&M University is poised to tackle the gamebird population crisis head-on.

Department of Health and Human Performance Shines at Annual Industry Conference
Faculty and students from the department received awards and presented workshops at the premier industry conference.
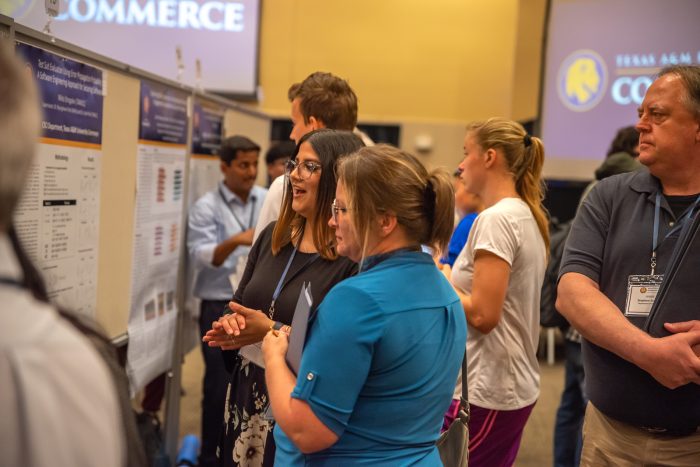
Showcasing Scholarship: Highlights from TAMUC’s Annual Research Symposium
TAMUC students present innovative research projects to the university community and industry leaders. Texas A&M University-Commerce hosted its Annual Research Symposi…
Meet our Team
Contact Us
- Division of Research and Economic Development
- 903.886.5964
- 903.468.8784
- Nursing and Health Sciences 320
- 2600 South Neal Street
- Commerce, TX 75428
- P.O. Box 3011
- Commerce, TX 75429-3011



 Sponsored Programs
Sponsored Programs



