
- On this page:
-
 Projects
Projects
The Development of Synthetic Hosts for Environmental and Biological Applications
Improving Safety
Using both computational and synthetic techniques, the Starnes research group focuses on the detection of molecules, based on their negative charge (anions) or their three-dimensional shape (chirality). A receptor molecule can be optimized, to bind specifically to a target, in order to indicate detection. The ability to identify the presence of target molecules, whether toxic forever chemicals or a desired shape of a medicinal drug, can lead to increased safety or improvement in efficacy.
Projects
There are two main projects in the Starnes research group.
Project 1
The Development of Synthetic Hosts for Environmental Contaminants and Anions of Biological Significance
The Starnes research group is centered on developing synthetic receptors for anions of environmental and biological significance.1 Environmentally, many anions (such as perchlorate, nitrate, nitrite, sulfate and pertechnetate) present themselves as toxic and problematic contaminants in lakes, rivers, aquifers, nuclear waste repositories, etc. Recently, a new class of environmental contaminants known as perfluoroalkyl and poly-fluoroalkyl substances (PFOS) have emerged, which are persistent organic pollutants. They persist in the environment and bioaccumulate and may be associated with health problems. 2 Our goal is the development of sensors and extraction agents for these compounds.
Many anions of biological importance exist, such as metabolites, DNA, RNA, proteins and peptides. Developing receptors for these analytes has diagnostic applications in drug delivery and monitoring cellular processes.
The research utilizes computational software to design the artificial receptor on a computer, analyze its conformational preferences computationally and then evaluate the receptors’ molecular recognition properties computationally. Receptors showing promise computationally are then synthesized in the lab and studied for their anion recognition properties.
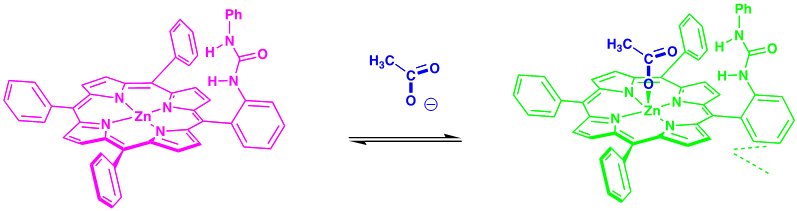
Representative example of anion recognition
Project 2
The Development of Synthetic Hosts for Chiral Recognition Applications
The research group is working on modifying hosts previously prepared in the research group that have been shown to function as stereoselective hosts for chiral guests3,4 to
- improve on the selectivity of these types of hosts in their guest binding properties and
- to learn more about the conformations of the hosts and host-guest complexes which will allow the group to improve on host design.
One practical result of the work is that it will lead to a better understanding of biological chemistry. Chiral compounds are essential, especially in biological chemistry. For example, one enantiomer of a chiral drug is useful, whereas its enantiomer might be toxic or deadly. Many biological substrates and structures are chiral (such as proteins and what they act on or the product of an enzyme-catalyzed reaction). By understanding chiral recognition better, we can better appreciate biological chemistry or biological recognition. Understanding the structures of the hosts and their complexes will contribute to a better understanding of the requirements for selective chiral recognition. The research could also impact the design of sensors for chiral species, the development of catalysts for chiral synthesis and the separations industry (for the separation of chiral substances such as enantiomeric molecules, which would significantly impact the pharmaceutical industry since one enantiomeric of a chiral drug might be toxic and therefore must be isolated and removed from the drug mixture).
A student working on these projects will be trained in synthetic organic chemistry, including the synthesis, isolation, purification and identification of organic compounds. The student will study the systems using computational chemistry, NMR, IR, circular dichroism, fluorescence and mass spectrometry.


